neuer Blog
Im Bereich Polymeranwendungen , Schwindung Die Rate ist ein kritischer Parameter, der die Maßgenauigkeit, Leistung und endgültige Anwendungsergebnisse von Polymerprodukten.
Ob bei alltäglichen Kunststoffartikeln oder Präzisionskomponenten in fortschrittlichen Industriezweigen: Das Verständnis und die Kontrolle der Schrumpfung von Polymermaterialien sind für die Gewährleistung der Produktqualität und -funktionalität von entscheidender Bedeutung.
In diesem Artikel werden wir uns mit der Schlüsselfaktoren Einfluss auf die Polymerschrumpfung und Erforschung wirksame Methoden um das Schrumpfen zu reduzieren.
1. Definition der Schrumpfrate in Polymermaterialien
Die Schrumpfungsrate von Polymermaterialien bezeichnet den prozentualen Unterschied zwischen den Abmessungen eines Kunststoffteils bei Formtemperatur und seinen Abmessungen nach dem Entformen und Abkühlen auf Raumtemperatur. Sie spiegelt direkt den Grad der Dimensionsreduzierung des Kunststoffteils nach dem Abkühlen außerhalb der Form wider.
Zur Veranschaulichung: Man legt ein Kunststoffmodell bei hoher Temperatur in eine Form. Nach dem Abkühlen ist es kleiner als bei der Formtemperatur in der Form. Dieses Reduktionsverhältnis wird als Schrumpfungsrate bezeichnet.
Die Schrumpfungsrate wird nach der Formel berechnet:
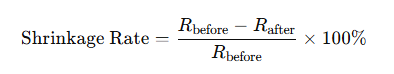
wobei R vorher die Abmessung bei der Formtemperatur und R nachher die Abmessung nach dem Abkühlen auf Raumtemperatur ist.
Aufgrund unterschiedlicher Molekülstrukturen, chemischer Zusammensetzungen und anderer Faktoren weisen verschiedene Polymermaterialien unterschiedliche Schrumpfraten auf. Daher ist die Berücksichtigung der Schrumpfrate bei der Materialauswahl und der Gestaltung des Formgebungsprozesses besonders wichtig.
2. Faktoren, die die Schrumpfrate von Polymermaterialien beeinflussen
Einfluss der Rohstoffe
1. Verschiedene Arten von Rohstoffen
Die Schrumpfungsraten variieren erheblich zwischen verschiedenen Arten von Polymermaterialien.
Beispielsweise weisen langfaserverstärkte modifizierte Materialien typischerweise eine Schrumpfrate von etwa 1,2 % bis 1,5 % auf.

PP-Homopolymer, 40 % langglasfaserverstärkt
2.
Kristallinität der Rohstoffe
Bei einem Material mit hohem Kristallinitätsgrad sind die Molekülketten dicht und geordnet angeordnet. Beim Abkühlen wechseln die Ketten vom ungeordneten geschmolzenen in den geordneten kristallinen Zustand, was zu einer deutlichen Schrumpfung führt. Die dicht gepackten Ketten in den kristallinen Bereichen reduzieren das Volumen des Materials, was zu einer höheren Schrumpfrate führt. Im Gegensatz dazu weisen amorphe (nicht-kristalline) Materialien im Allgemeinen eine geringere Schrumpfung auf.
Eine einfache Analogie ist das Stapeln von Holz: Zufällig gestapeltes Holz nimmt mehr Platz ein, während ordentlich gestapeltes Holz weniger Volumen einnimmt.

3.
Molekulargewicht der Rohstoffe
Bei hohem Molekulargewicht sind die intermolekularen Kräfte zwischen den Ketten stärker, was die Kettenbewegung erschwert. Beim Formen und Abkühlen können sich die Ketten nicht so leicht neu anordnen und dicht packen, was zu einer geringeren Schrumpfungsrate führt. Materialien mit niedrigerem Molekulargewicht hingegen haben beweglichere Ketten, die sich beim Abkühlen leichter neu anordnen und aggregieren können, was zu einer höheren Schrumpfungsrate führt.
Einfluss des Formprozesses
1.
Formtemperatur
Eine höhere Formtemperatur erhöht die Beweglichkeit der Molekülketten und verbessert den Schmelzfluss, sodass mehr geschmolzenes Material in den Formhohlraum gelangt. Höhere Temperaturen erhöhen jedoch auch die Schrumpfungskraft beim Abkühlen. Andererseits ermöglicht die längere Angussversiegelungszeit bei höheren Temperaturen, dass mehr Schmelze in den Hohlraum gelangt, was die Formdichte erhöht. Insgesamt führt dieser kombinierte Effekt oft zu einer geringeren Schrumpfungsrate.
2.
Haltedruck
Ein höherer Nachdruck beim Formen komprimiert mehr Schmelze in die Kavität, gleicht die durch die Abkühlungsschrumpfung verursachte Volumenreduzierung aus und reduziert so die Schrumpfungsrate. Bei kristallinen Materialien zeigt der Nachdruck eine kurvenförmige Abwärtstendenz, während bei einigen amorphen Materialien die Schrumpfungsrate mit zunehmendem Nachdruck linear abnimmt.
3.
Schmelztemperatur
Eine höhere Schmelztemperatur erhöht die molekulare Wärmebewegung und verbessert die Fließfähigkeit, wodurch die vollständige Füllung des Formhohlraums erleichtert wird. Bei kristallinen Materialien kann sie auch das Kristallisationsverhalten und damit die Schwindung beeinflussen. Im Allgemeinen verringert eine höhere Schmelztemperatur die Schwindung, eine zu hohe Temperatur kann jedoch zu Materialzersetzung führen.
4.
Formtemperatur
Niedrigere Formtemperaturen führen dazu, dass die Schmelze schneller erstarrt, was die Abkühlzeit verkürzt und Schrumpfungsreaktionen reduziert, wodurch die Schrumpfungsrate sinkt. Höhere Formtemperaturen verlangsamen die Abkühlung, wodurch eine stärkere Schrumpfung möglich wird.
5.
Haltezeit
Eine längere Haltezeit ermöglicht es der Schmelze, die durch Abkühlungsschrumpfung unter Druck entstandenen Lücken weiter zu füllen, wodurch die Schrumpfungsrate reduziert wird. Eine zu lange Haltezeit kann jedoch den Formzyklus verlängern und zu Spannungskonzentrationen im Produkt führen.
6.
Abkühlzeit in der Form
Bei großen Kunststoffprodukten mit dicken Wänden ist eine ausreichende Kühlzeit in der Form erforderlich, um eine vollständige Abkühlung und Verfestigung zu gewährleisten. Dies trägt zur Stabilisierung der Molekülketten in ihrer endgültigen Anordnung bei, was zu einer stabilen oder sogar reduzierten Schrumpfung führt. Eine unzureichende Kühlzeit kann nach dem Entformen zu weiterer Schrumpfung und damit zu Maßabweichungen führen.
7.
Injektionsgeschwindigkeit
Bei dünnwandigen Produkten ermöglicht eine höhere Einspritzgeschwindigkeit ein schnelles Füllen der Kavität durch die Schmelze. Dies kann jedoch zu höheren Scherspannungen führen, die eine Orientierung der Molekülketten und anisotrope Schrumpfung zur Folge haben. Eine geringere Einspritzgeschwindigkeit kann zu einer ungleichmäßigen Füllung führen, was sich ebenfalls auf die Schrumpfung auswirkt.
Einfluss der Struktur
1.
Wandstärke von Kunststoffprodukten
Eine Erhöhung der Wandstärke verlangsamt die innere Abkühlungsrate, ermöglicht eine vollständigere Kristallisation und einen höheren Kristallinitätsgrad, was wiederum zu einer erhöhten Schrumpfung führt. Bei einigen amorphen Materialien zeigt der Einfluss der Wandstärke auf die Schrumpfung kein klares Muster.
2.
Vorhandensein von Einlegeteilen in Spritzgussteilen
Bei Spritzgussteilen mit Metalleinsätzen kann der Unterschied im Wärmeausdehnungskoeffizienten von Metall und Polymer zu einer ungleichmäßigen Schrumpfung beim Abkühlen führen und so zu inneren Spannungen führen. Dies kann zu einer ungleichmäßigen Schrumpfung um die Einsätze herum führen und so zu Verzug, Rissbildung oder anderen Defekten führen.
3.
Form des Spritzgussteils
Komplexe Formen – wie Teile mit Übergängen zwischen dünnen und dicken Wänden, Rippen, Vorsprüngen oder anderen Merkmalen – können zu ungleichmäßiger Schrumpfung führen. Dünne Abschnitte kühlen schnell ab und schrumpfen weniger, während dicke Abschnitte langsam abkühlen und stärker schrumpfen, wodurch Schrumpfungsunterschiede innerhalb desselben Teils entstehen. Asymmetrische Teile neigen zudem eher zu ungleichmäßiger Schrumpfung.
4.
Schrumpfung in Längen- vs. Dickenrichtung
Beim Spritzgießen sind die Molekülketten in der Schmelze tendenziell stärker in Fließrichtung (Längsrichtung) ausgerichtet, was zu einer relativ geringeren Schrumpfung führt. Senkrecht zur Fließrichtung (Dickenrichtung) sind die Molekülketten weniger ausgerichtet, was zu einer relativ höheren Schrumpfung führt.
Einfluss der Formstruktur
1.
Gate-Größe
Beim Spritzgießen verringert ein größerer Anguss den Fließwiderstand der Schmelze, ermöglicht den Eintritt von mehr Material in die Kavität, sorgt für eine gleichmäßigere Druckverteilung und erhöht die Formdichte, was zu einer geringeren Schrumpfungsrate führt. Umgekehrt erhöht ein kleinerer Anguss die Schrumpfung.
2.
Schrumpfung in Richtungen parallel und senkrecht zum Anguss
In Fließrichtung (parallel) sind die Molekülketten stärker ausgerichtet, was zu einer geringeren Schrumpfung beim Abkühlen führt. In der Richtung senkrecht zum Anguss ist die Ausrichtung der Molekülketten geringer, was zu einer stärkeren Schrumpfung und möglicherweise zu Verzug oder Verformung führt.
3.
Schrumpfungsunterschiede zwischen Bereichen in der Nähe und weiter vom Gate
Bei großen Formen ist der Schmelzedruck in den angussfernen Bereichen geringer, die Füllung erfolgt später und der Halteeffekt ist schwächer, was zu einer relativ höheren Schwindung führt. Angussnahe Bereiche weisen im Allgemeinen eine geringere Schwindung auf.
Modifizierungsmethoden zur Reduzierung der Schrumpfung von Polymermaterialien
Faserverstärkung
Am Beispiel von Polymilchsäure (PLA) kann die Zugabe von Kurzglasfasern die Formschrumpfung deutlich reduzieren. Ab einem bestimmten Glasfaseranteil nimmt die Schrumpfung von PLA-Verbundwerkstoffen deutlich ab. Im Vergleich zu Kurzglasfasern
langglasfaserverstärkte Polymere
ausstellen sogar
geringere Schrumpfung
, mit gleichmäßiger Schrumpfung in Längs- und Querrichtung. Whisker-verstärkte Polymere weisen ebenfalls eine geringe Schrumpfung auf; beispielsweise weist mit Calciumsulfat-Whiskern verstärktes PLA eine geringere Schrumpfung auf als mit Glasfasern verstärktes PLA.

Langfaserverstärkte Polymere